Overview

The Purpose Of The Trial
What is AI in MRI all about?
The Purpose Of The Trial
The AssistMS programme will explore how artificial intelligence (AI) can help doctors assess MRI scans and make treatment decisions for people with multiple sclerosis (MS). The aim is to improve care and outcomes for people living with the condition.
AssistMS is a joint project between Queen Mary University of London (QMUL), icometrix, and
The AssistMS programme will explore how artificial intelligence (AI) can help doctors assess MRI scans and make treatment decisions for people with multiple sclerosis (MS). The aim is to improve care and outcomes for people living with the condition.
AssistMS is a joint project between Queen Mary University of London (QMUL), icometrix, and the University of Nottingham. The trial will take place in hospitals linked to Barts Health NHS Trust, Nottingham University Hospitals NHS Trust, and University Hospitals Birmingham NHS Trust. QMUL is sponsoring the trial, with additional support from the East Midlands Imaging Network (EMRAD), InHealth Group, and the MS Society of Great Britain & Northern Ireland.
The project is fully funded by the National Institute for Health and Care Research (NIHR). It will focus on how neuroradiologists assess MRI scans as part of routine MS care. People with MS who are receiving disease-modifying treatments (DMTs) have annual MRI scans of the central nervous system to check for disease activity. These scans help doctors decide whether a person’s treatment is working or if a change is needed.
MRI scans can detect disease activity much earlier than physical symptoms. Spotting changes early can help prevent worsening disability by allowing doctors to adjust treatment in time. However, analysing MRI scans is complex, time-consuming, and can be prone to human error.
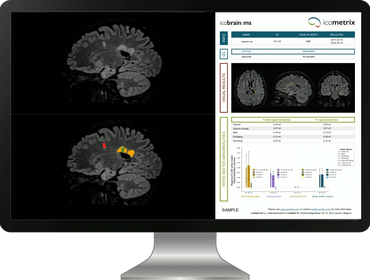
In the AssistMS trial, neuroradiologists and radiologists will assess MRI scans both with and without the support of icobrain ms, an AI tool designed to detect subtle changes in the brain.
The project will assess how accurate and reliable this AI tool is in identifying disease activity and other important clinical features in MRI scans from 1,336 people with MS in East London, Nottingham, and Birmingham.

Meet The Team
What is AI in MRI all about?
The Purpose Of The Trial
Professor Klaus Schmierer (aka Prof K), a specialist Neurologist in Multiple Sclerosis, at Queen Mary University London has partnered with MS centres in Nottingham and Birmingham to investigate this cutting edge technology.
Klaus Schmierer, Professor of Neurology at Queen Mary and joint lead of AssistMS, said: "I am thrilled about this
Professor Klaus Schmierer (aka Prof K), a specialist Neurologist in Multiple Sclerosis, at Queen Mary University London has partnered with MS centres in Nottingham and Birmingham to investigate this cutting edge technology.
Klaus Schmierer, Professor of Neurology at Queen Mary and joint lead of AssistMS, said: "I am thrilled about this generous award funded by NIHR through the NHS AI Lab's AI in Health and Care Award. If successful, AssistMS will have a significant impact on people with MS' quality of life as well as equity and efficiency of MS care across the UK."
Wim Van Hecke , CEO icometrix, said:
"We are extremely grateful and excited to share this prestigious Award with Queen Mary University of London, and especially being able to collaborate on the AssistMS programme pushing the boundaries of healthcare further through our validated and potentially cost-saving icobrain ms solution.
Our joint efforts will undoubtedly transform care and improve outcomes for many people with MS for the better across Europe and beyond."

What is AI in MRI all about?
What is AI in MRI all about?
What is AI in MRI all about?
Professor Schmierer has teamed up with icometrix, a world-leading company in neurology AI that specialises in multiple sclerosis (MS). They are providing the technology for the trial.
The software being tested in the AssistMS trial, called icobrain ms, analyses brain MRI scans to detect and highlight small changes over time. It also creat
Professor Schmierer has teamed up with icometrix, a world-leading company in neurology AI that specialises in multiple sclerosis (MS). They are providing the technology for the trial.
The software being tested in the AssistMS trial, called icobrain ms, analyses brain MRI scans to detect and highlight small changes over time. It also creates easy-to-read summary reports. The goal is to help doctors make better decisions about disease-modifying treatments (DMTs). By spotting signs of disease activity earlier and more accurately, clinicians can decide more quickly if a treatment change is needed.

Welcome to the AssistMS Trial Website
Queen Mary and icometrix have together been awarded a prestigious Artificial Intelligence (AI) Award in Health and Care by the National Institute for Health and Care Research (NIHR)
More about the trial

NIHR - Artificial Intelligence funding award
To assess the clinical usefulness, patient satisfaction and cost-effectiveness of an Artificial Intelligence (AI) tool called icobrain ms, and to test its implementation in routine care at three NHS Trusts.
Multiple sclerosis (MS) is a chronic, disabling disease driven by an abnormal immune response to the central nervous system. Over 120,000 people live with MS in the UK costing the NHS more than £1billiona year. Early disease modifying treatment (DMT) is part of the standard of care for people with MS (pwMS). Unless effectively treated, MS leads to significant disability, and associated care costs, in most cases. However, whether any of the currently licensed fifteen DMTs is effective in an individual person with MS is unpredictable. Effective treatment monitoring is essential to (i) detect signs of disease activity before the individual suffers its effects and (ii) enable early switching to a different, hopefully (more) effective, DMT.
In clinical practice, regular magnetic resonance imaging (MRI) is the only established tool for DMT efficacy monitoring. However, detecting the often subtle changes by inspecting MRI scans is time consuming, tiring and therefore error-prone. icobrain ms is a validated AI technology enabling quantification of MRI datasets, summarising findings in a structured electronic report as well as annotated images highlighting areas of change that help guide assessment. icobrain ms complements visual assessment of MRI scans and helps the clinician to decide whether or not a change in DMT is warranted.
Patient and public involvement: Assist-MS has been developed in partnership between neurologists, image-analysts and pwMS, reflecting the interest of pwMS to control their condition, including safe and effective DMT.
Our PPI leadDominic Shadbolt (aka @theMSguide) has relapsing MS undergoing regular MRI monitoring himself. Joint lead Prof Schmierer has strong links with MS Society, UK MS Register, MS Trust and www.theMSguide.com, which will all help continue a strong anchoring of Assist-MS in the needs of pwMS.
The results of Assist-MS will be presented at national and international meetings, and written up for peer-review. We will also provide summaries in lay language to communicate with pwMS and the wider public via our blog, the MS charities websites, Twitter and other social media.

More about MRI in MS
Optimizing MS Care Through AI-Driven MRI Analysis
Effective monitoring of sub-clinical disease activity is critical for managing multiple sclerosis (MS). While magnetic resonance imaging (MRI) remains the gold standard for detection, manual visual assessment of scans is time-consuming, prone to human error, and contributes to clinician fatigue.
icobrain ms—a validated AI-powered technology—complements traditional visual MRI analysis by automating lesion detection and quantifying disease activity with precision. By streamlining this process, it empowers clinicians to make timely, data-driven decisions about modifying Disease-Modifying Therapies (DMTs).
Trial Objectives:
- Superiority in Detection: Evaluate whether icobrain ms outperforms standard visual assessment in identifying active MS lesions.
- Health-Economic Impact: Analyze how AI integration reduces MRI analysis time, optimizes resource allocation, and improves long-term tracking of MS progression.
This dual focus aims to validate icobrain ms as both a clinically superior tool and a cost-effective solution for healthcare systems, ultimately enhancing personalised care for people with MS (pwMS).

Main Aims of the AssistMS Trial
The AssistMS trial focuses on three key objectives:
1 - Comparing AI and Human Radiologists in MRI Analysis
The trial aims to determine whether the icobrain ms software is more effective than standard visual assessment by radiologists in detecting MRI disease activity in people with multiple sclerosis (pwMS).
2 - Impact of AI-Detected Changes on Treatment Decisions
The study will assess whether pwMS who experience disease activity detected by standard radiologist review—and subsequently have their disease-modifying therapy (DMT) adjusted—show better clinical outcomes compared to those whose disease activity is identified only by icobrain ms but do not undergo a DMT change.
3 - Effect on Healthcare Resources
The trial will evaluate the impact of icobrain ms on healthcare efficiency, including the time required for MRI review, the speed from MRI acquisition to decision-making, the frequency and cost of DMT adjustments, and overall follow-up care needs.

icometrix are the technology partners for the trial. It is their AI MRI analytics tools that underpin the trial.
Frequently Asked Questions
Please reach us at assistms@qmul.ac.uk if you cannot find an answer to your question.
If you have agreed to take part then thank-you.
You will have been asked to return your forms withing 7 days. This really matters.
If you want to learn more then spend a few minutes watching this video: https://youtu.be/smYcRrdCVWg
The aim of the trial is to randomly assign participants into two groups.
- In the first group, MRIs will be analysed conventionally by a neuroradiologist, who will share their report with your MS treatment team.
- In the second group, the neuroradiologist will produce their usual report, but the scans will also be analysed by the icobrain software.
This additional AI analysis will be evaluated for its accuracy and to determine whether it speeds up the neuroradiologist’s workflow.
As someone living with MS, you likely undergo annual monitoring scans. We are asking for your permission to have your scans analysed by the icobrain ms AI tool, in addition to the standard report from the neuroradiologist.
Your participation requires no extra effort from you. The trial will only analyse the final scans after they have been completed. You won’t need any additional scans, and the length or type of your scan will remain unchanged.
This is a technology trial, not a drug trial. You will not be required to take any additional medication beyond what your neurologist has prescribed.
In short, no. If you take part in the AssistMS trial, you will be randomly assigned to one of two groups, and you won’t know whether your scans are being additionally analysed by the AI software.
This process, known as randomisation, helps eliminate as many potential sources of bias—whether intentional or accidental—as possible. This ensures the trial results are as accurate and reliable as possible.
Importantly, your care will not be affected in any way. Every scan will still be reviewed as usual by a specially trained human radiologist.
Download Trial Documents
Before signing up for the AssistMS trial, please download and read the Trial Documents here first.
Step Into Our World: The ASSIST-MS Photo Gallery
Contact the Assist MS team
ASSIST-MS Trial
